Chapter 15. Aldehydes and Ketones
Recommended Article: 【Organic Chemistry】 Organic Chemistry Table of Contents
1. Overview
2. Nucleophilic Addition Reactions
1. Overview
⑴ Nomenclature
① Aldehyde Nomenclature
○ Longest chain containing CHO is the parent
○ Aldehyde carbon in CHO is numbered 1
○ Aldehyde attached to a ring is parent: Named with suffix -cycloalkanecarbaldehyde
○ Aldehyde in a chain structure is treated as a substituent: Named with oxo
○ Aldehyde in a ring structure is treated as a substituent: Named with formyl
○ Comparison of systematic nomenclature (left) and common nomenclature (right)
○ methanal / formaldehyde
○ ethanal / acetaldehyde
○ 2-bromopropanal / α-bromopropionaldehyde
○ 3-chlorobutanal / β-chlorobutyraldehyde
○ 3-methylbutanal / isovaleraldehyde
○ hexandial /
② Ketone Nomenclature
○ Longest chain containing C=O is the parent
○ End of the chain closest to C=O is numbered 1
○ Ketone attached to a ring is parent: Named with suffix -cycloalkanone
○ Ketone in a chain or ring structure is treated as a substituent: Named with oxo
○ Comparison of systematic nomenclature (left) and common nomenclature (right)
○ propanone / acetone, dimethyl ketone
○ 3-hexanone / ethyl propyl ketone
○ 6-methyl-2-heptanone / isohexyl methyl ketone
○ 2,4-pentanedione / acetylacetone
○ cyclohexanone /
○ butanedione /
⑵ Common Reaction Principles
① Nucleophilic attack, Electrophilic attack
② Proton transfer: Occurs intermolecularly, not intramolecularly
③ Tautomeric equilibrium: Enol ↔ Ketone
④ Work-up: Useful keyword for purification without specifying exact acid or base
⑶ Reactivity Comparison
① Carbonyl compound reactivity comparison
○ acyl halide > acid anhydride > aldehyde > ketone > ester ~ carboxylic acid > amide > carboxylate ion
② Ketone reactivity comparison based on functional groups
Figure 1. Ketone reactivity comparison based on functional groups
○ Aldehydes are more reactive, electrophilic, and have higher combustion heat than ketones
○ Reason 1: Steric hindrance: More substituents hinder nucleophilic attack, reducing nucleophilicity
○ Reason 2: Inductive effect: Substituents provide electron density to ketone carbon via hyperconjugation
③ Other Comparisons
○ Reactions involving aldehydes and ketones are quite similar
○ Ketones are more polar than aldehydes
○ Reason: Ketones stabilize carbocations in resonance contributors by methyl groups
○ Aliphatic ketones are more reactive than aromatic ketones
○ Differences in reactivity between aldehydes and ketones are observed in oxidation-reduction reactions
○ Reason: Aldehydes can undergo further oxidation, whereas ketones cannot be further oxidized
○ Example: Silver mirror reaction, Fehling’s reaction, Benedict’s reaction only occur with aldehydes
2. Nucleophilic Addition Reactions
⑴ Overview
① Bürgi–Dunitz angle: Angle at which nucleophile’s HOMO approaches the antibonding orbital (LUMO) of the carbonyl group
② Acidic conditions: Increase carbonyl’s electrophilicity
③ Basic conditions: Increase nucleophilicity of the nucleophile
④ Halide ions do not act as nucleophiles toward carbonyl compounds
⑵ Type 1. When the Basicity of Y- is Greater than that of X-
① Overview
○ Type 1 is frequently observed in acyl chlorides
○ Carboxylic acids also often show behavior similar to Type 1
○ Cases of aldehydes and ketones reacting as Type 1 are rare
Figure 2. Carbonyl compound reaction Type 1
② Example 1. Acyl chloride: Basicity of Cl- is very low
Figure 3. Reaction of acyl chloride
⑶ Type 2. When the Basicity of Y- is Less than that of X-
① Overview
○ Aldehydes and ketones mostly follow Type 2
○ Other reactions often involve applications of Type 2
Figure 4. Carbonyl compound reaction Type 2
② Example 1. Acid-catalyzed carbonyl addition reaction
Figure 5. Acid-catalyzed carbonyl addition reaction
③ Example 2. Base-catalyzed carbonyl addition reaction
Figure 6. Base-catalyzed carbonyl addition reaction
④ Example 3. Acetal Formation Reaction: Carbonyl Carbon Protection Reaction
○ Acetal and hemiacetal

Figure 7. Structures of hemiacetal, acetal, hemiketal, and ketal
○ Reaction Mechanism : (Formula) Remove ketone oxygen and attach two -OR groups
Figure 8. Example of acetal formation reaction
○ Acetal formation reaction has reverse reaction under the same conditions
○ Dean-stark trap removes H2O, increasing yield since H2O is a product of the reaction
○ Solvent: Azeotropic solvents like toluene, xylene with high boiling points and constant boiling points are used ( ∵ Dean-stark trap necessary condition)
○ Excess alcohol as a reactant increases yield → usually added in excess instead of 2 equivalents
○ Esters, carboxylic acids, and amides do not form acetals under typical acidic conditions
○ Example 3-1.
Figure 9. Acetal formation reaction
○ Example 3-2.
Figure 10. Acetal formation reaction
○ Example 3-3. Corey-Seebach reaction: Thiol addition reaction
Figure 11. Thiol addition reaction
○ Reduction reaction: thioacetal + H2 / Raney-Ni (desulfurization)
④ Example 4. Amine addition reaction
○ Mechanism: (Formula) Remove carbonyl (=O) and attach imine (=NR) instead
○ Since the reverse reaction is also possible, equilibrium is reached at a certain point.
○ Imines form best at pH 4-5
○ Example 4-1. General amine addition reaction
Figure 12. Aldehyde and ketone amine addition reaction
○ Example 4-2. p-toluenesulfonyl hydrazide (CH3-Ph-SO2NHNH2)
○ Example 4-3. Enamine formation reaction

Figure 13. Enamine formation reaction
○ Secondary amines lack a proton to remove from the iminium ion, unlike primary amines, so the hydrogen attached to the alpha carbon is removed.
○ Since C=N bond strength is greater than C=C bond strength, imine formation occurs during primary amine addition
○ Stork-enamine reaction: Enamines react as nucleophiles with alkyl halides, acyl halides, carbonyl compounds, etc.
○ Example 4-4. Reduction reaction of NaBH3CN: Imines are reduced to amines
Figure 14. Reduction reaction of NaBH3CN
○ Example 4-5. Circular RNA synthesis: Amine addition + NaBH3CN reduction
Figure 15. Circular RNA synthesis
○ Example 4-6. Wolff-Kishner reduction reaction
○ NH2NH2, strong base (e.g., KOH, NaOH), heating conditions induce additional reduction
○ When treating -C=N-NH2 (hydrazone) with base
○ Example 4-7. Beckmann rearrangement
○ When treating -C=N-OH (oxime) with an acid
○ Example 4-8. Pomeranz-Fritsch reaction

Figure 16. Pomeranz-Fritsch reaction
○ benzaldehyde + 2,2-dialkoxyethylamine for amine addition reaction and electrophilic aromatic substitution (EAS)
○ Example 4-9. Pictet-Spengler reaction

Figure 17. Pictet-Spengler reaction
○ Amine addition reaction and Friedel-Crafts alkylation
⑤ Example 5. HCN Addition Reaction of Aldehydes and Ketones
Figure 18. Mechanism of HCN addition reaction of aldehydes and ketones
○ Example 5-1. Benzoin condensation reaction
○ Example 5-2. Stetter Reaction
⑷ Type 3. Enone and 1,2-addition reaction
① Applies when the nucleophile is a strong nucleophile (e.g.: Grignard reagent)
② Mechanism
Figure 19. Mechanism of enone and 1,2-addition reaction
⑸ Type 4. Enone and 1,4-addition reaction (Michael addition)
① Applies when the nucleophile is a weak nucleophile (e.g.: MeSH)
② Mechanism
Figure 20. Mechanism of enone and 1,4-addition reaction
③ Exception: In the case of acyl chlorides, 1,2-addition reaction occurs even with a weak nucleophile
Figure 21. Enone and acyl chloride
3. Other Reactions
⑴ Hydrogenation reaction
① Example 1. Hydrogenation with metal reagents
Figure 22. Hydrogenation of aldehydes and ketones by addition of metal reagents
② Example 2. Pd/C hydrogenation reaction: Alkene is more reactive than ketone
Figure 23. Pd/C hydrogenation under H2 (1 eq.) conditions
Figure 24. Pd/C hydrogenation under H2 (excess) conditions
⑵ Oxidation-reduction reaction
① Example 1. Organic metal reagent reaction with aldehydes and ketones
○ When there is an alkene and a ketone, the organic metal reagent reacts with the alkene first
Figure 25. Reaction of aldehydes and ketones with organic metal reagents
② Example 2. Wittig reaction: Addition reaction of ylide to aldehydes and ketones
○ Ylide reagent
○ Ylide refers to a molecule with adjacent atoms having opposite polarity
○ When the P=C bond forms, the inner d orbital electrons of the P atom are involved in bonding, resulting in high energy, and the zwitterion ylide, which is a separated charge, becomes the actual structure
Figure 26. Ylide reagent
○ Preparation of ylide reagents: 1. Ph3P, CH3Br, 2. BuLi
Figure 27. Preparation of ylide reagents
○ Example 2-1. (General) O of the ketone is replaced with C
Figure 28. Wittig reaction example
○ Example 2-2.

Figure 29. Wittig reaction example
○ Advantages: Alkene with fewer substituents becomes the sole product
○ Disadvantages: Wittig reaction generates alkenes without (E)/(Z) selectivity, while Figure 27. does show selectivity, making it worth noting
○ Solution
○ If the ylide nucleophile’s carbon is attached to an electron-withdrawing group (EWG), the reactivity is low, so thermodynamically stable alkene is predominantly formed.
○ If the ylide nucleophile’s carbon is attached to a phenyl group, the kinetically favored (less sterically hindered) alkene is predominantly formed.
③ Example 3. Horner-Wadsworth-Emmons reaction
○ Selective reaction to obtain (E)-alkenes

Figure 30. Mechanism of Horner-Wadsworth-Emmons reaction
④ Example 4. Reformatsky reaction
○ Addition reaction with organic zinc reagents
○ Organic zinc reagents are less reactive and do not react with ester groups: Unlike Grignard reagents and esters
○ After formation of the Reformatsky reagent, aldehydes and ketones undergo addition reaction

Figure 31. Reformatsky reaction
⑤ Example 5. Cannizzaro reaction
○ Intermolecular oxidation-reduction reaction of aldehydes without α-hydrogens using strong base
○ benzaldehyde + KOH + H2O → potassium benzoate (oxidized) + benzyl alcohol (reduced)
Figure 32. Mechanism of Cannizzaro reaction
⑥ Example 6. Meerwein-Ponndorf-Verley reduction (Oppenauer oxidation)
○ Reduction of carbonyl group and oxidation of alcohol catalyzed by Al(OC(CH3)3)3
○ Opposite reaction is called Oppenauer oxidation
Figure 33. Meerwein-Ponndorf-Verley reduction (Oppenauer oxidation)
⑶ Precipitation reaction: Aldehydes can be further oxidized while ketones cannot, resulting in a difference in precipitation reaction
① Silver mirror reaction (Tollens test)
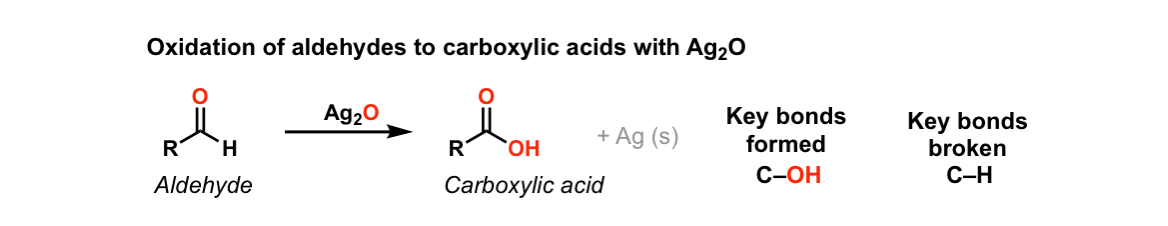
Figure 34. Silver mirror reaction
○ Tollens reagent: Colorless solution
○ Step 1. Dissolve AgNO3 in water
○ Step 2. Add NaOH or KOH to precipitate Ag as Ag2O
○ Step 3. Add ammonia solution to form silver-ammonia complex ion Ag(NH3)2+NO3-
○ Experimental process
○ Step 1. Add reducing substance to silver nitrate ammonia solution (Tollens reagent) and heat
○ Step 2. Reduction of silver ion, which was in ion form, leads to the precipitation of Ag(s)
○ Reducing sugar: Formation of metallic silver
○ Aldehyde: Formation of metallic silver
○ Ag2O acts as an Ag+ donor: oxygen in water moves to an aldehyde, resulting in the creation of a carboxylic acid.
○ Ketone: No reaction
○ Exception: Reacts with α-hydroxy ketones
○ Acetal: No reaction
○ Hemiacetal: Reaction
○ Reason: Hemiacetals can be cleaved into carbonyl and alcohol groups
② Fehling reaction
○ Fehling’s reagent: Copper sulfate pentahydrate (CuSO4·5H2O), NaOH, Rochelle salt (sodium potassium tartrate)
○ Experimental process
○ Step 1. Add reducing substance to Fehling’s solution and heat
○ Step 2. Reduction of copper ion, which was in ion form with tartaric acid, leads to the precipitation of copper(I) oxide (Cu2O)
○ Reducing sugar: Formation of copper precipitate (red-brown)
○ Aliphatic aldehyde: Formation of copper precipitate (red-brown)
○ Aromatic aldehyde: No reaction
○ Ketone: No reaction
③ Benedict’s reaction
○ Benedict’s solution: CuSO4, Na2CO3, sodium citrate
○ Aldehyde: Formation of copper precipitate (red-brown)
○ Ketone: No reaction
○ Does not oxidize creatine and uric acid
○ Oxidizes glucose, fructose, and maltose, etc.: Convenient for detection reactions
⑷ Rearrangement reactions
① Example 1. Baeyer-Villiger oxidation: Mechanism of seven-membered ring structure
○ Overview
○ Under peroxy acid, excess oxygen is transferred to an aldehyde (or ketone) to produce a carboxylic acid (or ester).
○ Retention of configuration during rearrangement.
○ Migratory aptitude
○ H >phenyl > tertiary alkyl > cyclohexyl > secondary alkyl > primary alkyl > methyl
○ Oxygen insertion occurs towards electron-rich carbon (ref)
○ 1-1. acetophenone: Benzene ring enhances reactivity
○ Acetophenone reacts with peroxidic acid (RO3H), e.g. CF3CO3H, to form phenyl acetate
Figure 35. Mechanism of Baeyer-Villiger oxidation
Considering the hydrogen of -OH, it can be seen that a heptagon is formed
○ 1-2. _m_CPBA
○ _m_CPBA in the form of RCO3H oxidizes ketones
○ Oxygen insertion occurs towards electron-rich carbon
○ Competes with epoxidation of alkenes, but Baeyer-Villiger reaction is dominant
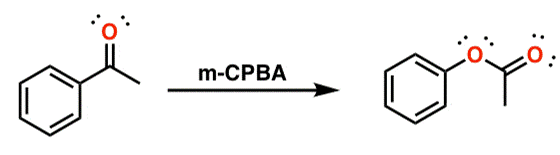
Figure 36. _m_CPBA catalyzed Baeyer-Villiger oxidation
② Example 2. Tiffeneau-Demjanov rearrangement
○ Formation of alkyl diazonium salt leads to instability and formation of carbocation
Figure 37. Mechanism of Tiffeneau-Demjanov rearrangement
③ Example 3. Johnson-Corey-Chaykovsky reaction
○ Example 3-1. Johnson-Corey-Chaykovsky reaction using sulfur ylide
○ Various types of sulfur ylides are as follows:
○ H2C-S(CH3)2: sulfur ylide (sulfonium)
○ H2C-SO-(CH3)2: Corey’s ylide (sulfoxonium)
Figure 38. Johnson-Corey-Chaykovsky reaction mechanism
○ Example 3-2. Johnson-Corey-Chaykovsky reaction of ketone under AlCl3, CH2N2 conditions
Figure 39. Johnson-Corey-Chaykovsky reaction of ketone under AlCl3, CH2N2 conditions
○ Example 3-3. Due to the strong nucleophilicity of sulfonium ylide, 1,2-addition is dominant
Figure 40. 1,2-addition of sulfonium ylide
○ Example 3-4. Since sulfoxonium ylide is a weak nucleophile (∵ ketone group), 1,4-addition is dominant
Figure 41. 1,4-addition of sulfoxonium ylide
> ④ Example 4. Benzilic acid rearrangement
Figure 42. Mechanism of benzilic acid rearrangement
> ⑤ Example 5. Favorskii rearrangement reaction
○ (Formula) Strong base, ketone, increase in unsaturation by one, removal of two leaving groups
Figure 43. Favorskii rearrangement reaction
4. Aldehyde Synthesis Methods
⑴ Ozonolysis of alkenes: 1. O3, 2. Zn, H2O or CH3SCH3
⑵ Hydration of terminal alkynes: 1. BH3, 2. H2O2, OH-
⑶ Synthesis of benzaldehyde via Gattermann-Koch reaction: It is a kind of Friedel-Crafts acylation for formyl group.
⑷ Reaction between benzyl chloride and nitromethane anion: benzyl chloride + nitromethane anion → benzaldehyde
⑸ Weak oxidation of primary alcohols: Oxidized to aldehydes.
⑹ HIO4 oxidative cleavage of diols: HIO4, H2SO4
⑺ Reduction of acyl chlorides, esters, and nitriles
⑻ Reimer-Tiemann reaction: CHCl3, KOH
⑼ Corey-Seebach reaction: thioacetal + HgCl2, MeOH, H2O → aldehyde
① Role of sulfur atom: Reduction of negative charge through backbonding effect. Sulfur atom is easily polarized and stabilizes negative ions.
② Role of Hg2+: Lewis acid, solvent with hexagonal copper structure
③ Without Hg2+, the reverse reaction becomes dominant
5. Ketone Synthesis Methods
① Overview: An alkyne with an -OH group undergoes hydration to form an enol, which then undergoes tautomerization to produce a ketone.
② Acid-catalyzed hydration reaction: H2O, H2SO4 / H2O, H2SO4, HgSO4
③ Hydroboration-oxidation reaction: 1. BH3, 2. H2O2, OH- / 1. BH3, 2. H2O2, OH-
⑶ Friedel-Crafts acylation

Figure 44. Friedel-Crafts acylation of benzene
⑷ Oxidation of secondary alcohols
⑸ HIO4 oxidative cleavage of diols: 1. HIO4, 2. H2SO4
⑹ Corey-Seebach reaction: thioketal + HgCl2, MeOH, H2O → aldehyde
① Role of sulfur atom: Reduction of negative charge through backbonding effect. Sulfur atom is easily polarized and stabilizes negative ions.
② Role of Hg2+: Lewis acid, Solvation of hexagonal copper structure
③ Without Hg2+, the reverse reaction becomes dominant
⑺ Reaction of carboxylic acids with organolithium reagents
① Grignard and Gilman reagents do not react with carboxylic acids
⑻ Reaction of acyl chlorides with organometallic reagents
⑼ Reaction of nitriles with organometallic reagents
⑽ Imines’ acid-catalyzed hydration reaction
Input: 2019.03.29 13:54
Modification: 2023.01.30 23:12
































