Chapter 14. Epoxides
Recommended Post: 【Organic Chemistry】 Organic Chemistry Table of Contents
1. Nomenclature
2. Reactions
1. Nomenclature
⑴ Oxa Type
① Named as cycloalkane at the end
② Oxygen forming the ring is labeled as 1
③ The carbon with the most substituents is labeled as 2, and if the numbers are the same, alphabetical order is followed
⑵ Oxirane Type
① Named as oxirane at the end
② Oxygen forming the ring is labeled as 1
③ The carbon with the most substituents is labeled as 2, and if the numbers are the same, alphabetical order is followed
④ Example: 2-methyl-3-phenyloxirane
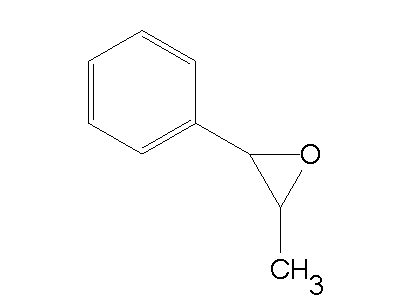
Figure 1. 2-methyl-3-phenyloxirane
⑶ Epoxyalkane Type
① Terminal carbon is labeled as 1
② If connected to the 3rd and 4th carbons, named as 3,4-epoxy-
③ Substituents are named in alphabetical order after the numbers are determined
⑷ Alkene Oxide Type
① Terminal carbon of the alkene is numbered to have the lower number
② Substituents are named in alphabetical order after the numbers are determined
2. Reactions
⑴ Overview
① Ring-opening reactions
② Epoxides are more reactive than ethers due to ring strain
⑵ Acid-Catalyzed Ring-Opening Reaction: Proceeds similar to SN1 reaction
① Mechanism

Figure 2. Acid-Catalyzed Epoxide Ring-Opening Reaction
② (Formula) Can be understood as an SN1-like reaction where initially, oxygen captures a proton, creating a carbocation on the carbon with more substitution
⑶ Base-Catalyzed Ring-Opening Reaction: Proceeds similar to SN2 reaction
① Mechanism

Figure 3. Base-Catalyzed Epoxide Ring-Opening Reaction
② (Formula) Strong nucleophile attacks, avoiding steric hindrance
⑷ Fürst-Plattner Rule
① For cyclic carbon compounds, the ring opens such that the -OH group and the nucleophile become trans axial.
② The ring opens in the axial direction of the chair form but not in the axial direction of the twist boat form.
③ Reason for trans axial conformation: Related to backside attack
⑸ Grignard Reagent Ring-Opening Reaction
① Mechanism

Figure 4. Grignard Reagent Ring-Opening Reaction
② (Formula) Strong nucleophile attacks, avoiding steric hindrance
3. Synthesis Methods
⑴ Alkene + Peroxycarboxylic Acid
⑶ Halohydrin Formation Reaction + Intramolecular SN2 Reaction

Figure 5. Epoxide Synthesis through Halohydrin Pathway
⑷ Johnson-Corey-Chaykovsky Reaction
Input: 2020.03.04 00:57
Modified: 2022.02.02 01:54